FAQs

Transcatheter aortic valve replacement (TAVR) is a minimally invasive procedure to replace the aortic valve in patients with heart valve failure (severe aortic stenosis†). TAVR is less invasive than open-heart surgery, and the procedure typically takes less than one hour. Your heart team will determine if you should have a mild sedative or general anesthesia.
At the start of the procedure, your doctor will make a small cut in one of three typically used access routes: a small cut in the groin (1), the neck (2), or a space between your ribs (3).
The doctor will guide a thin, flexible tube with the heart valve into your artery and to your diseased valve. Throughout the procedure, your doctor will be viewing images of your heart.
The Medtronic TAVR heart valve will be placed in your diseased valve. Your new valve will work immediately. Your doctor will remove the tube and close the cut.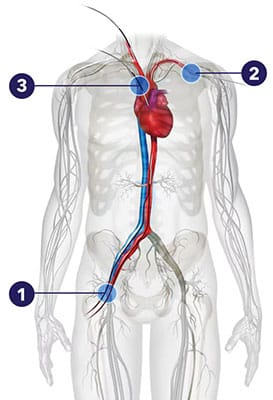
Actually, both men and women can benefit from the TAVR procedure and the use of Medtronic TAVR valve, Evolut™. The reason for pointing out Evolut’s value to women is that a recent clinical study showed superior valve performance‡ for women receiving Evolut compared to the SAPIEN TAVR valve.1* This study, the SMART Trial as it’s commonly known, focuses on health outcomes of patients with small heart valves, of whom approximately 87% were female.
While some patients take longer to feel better, most TAVR patients start noticing a difference right away. This is because their heart valve is now working properly. Some of the most commonly reported TAVR patient benefits include:
Designed to work like your own heart valve, the Medtronic TAVR heart valve offers a number of features that make it worth discussing with your heart team:
The Medtronic TAVR valve cannot be used for patients who:


† Based on the 1 year follow-up results from the SMART clinical trial which showed differences in valve performance‡ for Evolut™ compared to SAPIEN™* and no differences in safety outcomes. SMART primarily studied small annulus patients, predominantly women1. Additional clinical trials on women, regardless of their annulus size, have shown comparable mortality rates in women and men treated with TAVR2 and lower mortality rates for women treated with TAVR compared to women treated with surgical valve replacement3 at 1 year after the procedure.
‡ Valve performance is as defined as freedom from bioprosthetic valve dysfunction (BVD) through 12 months. BVD is defined as a composite including any of the following: hemodynamic structural valve dysfunction (mean gradient ≥ 20 mmHg), non-structural valve dysfunction (severe prothesis-patient mismatch or ≥ moderate aortic regurgitation), clinical thrombosis, endocarditis, and aortic valve reintervention.
§ Medtronic TAVR is indicated to treat patients who have been diagnosed with symptomatic severe aortic stenosis.
◊ Distinct advantages over SAVR include better blood flow from improved hemodynamics.
¶ Better blood flow, or “near-normal transvalvular flow” means that your new heart is operating well and the gradient across your valve is < 20 mmHg.
# Quality of life was based on results from the Kansas City Cardiomyopathy Questionnaire.
TAVR risks may include, but are not limited to, death, stroke, damage to the arteries, bleeding, and need for permanent pacemaker.